Catalysts - how do they work and what do they do?
"We reveal all about Catalysts in Car exhausts."

What is a catalyst and what does it do?
Some mistake the catalyst for a filter, it is clearly not. It merely causes a reaction in the exhaust gases that turn harmful emissions into more friendly ones.
In an ideal world a car engine would efficiently burn all the petrol supplied to the engine and emit nothing but carbon dioxide and water vapour.
Sadly, the internal combustion engine is not a very efficient beast, as a result several different pollutants are emitted in the exhaust gases.
A catalyst in an exhaust system is designed to help reduce harmful emissions that are produced by the engine during the combustion process. The catalyst works by promoting a chemical reaction that converts the pollutants in the exhaust gas into less harmful substances before they are released into the environment.
What Is Contained in a Typical Catalyst
The catalyst is typically made up of a combination of precious metals, such as platinum, palladium, and rhodium, that are coated onto a ceramic or metallic substrate in the form of a lattice.

How Do Catalysts Work?
When the exhaust gas passes through the catalyst, the pollutants, such as carbon monoxide, hydrocarbons, and nitrogen oxides, react with the metals on the surface of the catalyst.
These are then converted into carbon dioxide, water vapor, and nitrogen gas, which are less harmful to the environment.
Overall, the catalyst helps to reduce the amount of pollutants that are released into the air, helping to improve air quality and reduce the environmental impact of vehicles and other sources of combustion emissions.
When Did Catalysts Become A Requirement?
Catalytic converters were made mandatory in the UK for all new cars from 1st January 1993 in order to reduce pollution. They are frequently blamed for a loss of power which is something we address in our sports catalyst article.
The first automotive catalysts were introduced in the United States in the mid-1970s as a response to the implementation of the Clean Air Act, which established stricter emissions standards for vehicles. The first catalysts were designed to reduce emissions of carbon monoxide (CO) and hydrocarbons (HC) from gasoline engines.
The catalysts used in the early years were primarily made of a ceramic substrate coated with a precious metal, such as platinum or palladium, that facilitated the chemical reactions that reduced emissions. Over time, catalysts have become more advanced, with the addition of more sophisticated materials and designs to further improve their efficiency and durability.
Today, catalysts are a standard feature in most gasoline and diesel-powered vehicles sold in the United States and many other countries around the world. They are an essential component of modern emissions control systems and are necessary to meet the increasingly stringent emissions standards that have been put in place to protect the environment and public health.
What are the harmful components in exhaust fumes?
The incomplete combustion of the petrol gives rise to carbon monoxide and various Volatile Organic Compounds (VOCs).
This problem is worst when idling or decelerating. Carbon monoxide is poisonous and a greenhouse gas.
Volatile Organic Compounds are harmful to health and some are linked to cancer.
The very high temperatures in the engine (over 1500°C) causes nitrogen from the air to react with the oxygen from the air to form nitrous oxides.
These cause various problems, nitrogen dioxide for example reacts with the water in the atmosphere to form dilute nitric acid which gives rise to acid rain.
The action of sunlight on this mix of pollutants gives rise to photochemical smogs and the formation of other pollutants such as ozone.
So how does the catalyst convert these elements?
You can think of your catalytic converter as a dating agency for exhaust pollutants, helping them meet their ideal partner and converting them to carbon dioxide and water vapour.
Some of the chemical reactions that take place in the catalytic converter are shown below.
- carbon monoxide + oxygen → carbon dioxide
- Volatile Organic Compounds (partly burnt petrol) + oxygen → carbon dioxide + water
- nitrogen monoxide + carbon monoxide → carbon dioxide + nitrogen

The chemical processes that occur inside a catalyst can vary depending on the specific catalyst design and the pollutants that it is designed to reduce. However, in general, the following chemical processes occur in a catalyst:
- Oxidation: Carbon monoxide (CO) and hydrocarbons (HC) are oxidized by the catalyst, which means that they react with oxygen (O2) in the air to form carbon dioxide (CO2) and water (H2O). This reaction occurs on the surface of the catalyst and is facilitated by the presence of the precious metals.
- Reduction: Nitrogen oxides (NOx) are reduced by the catalyst, which means that they react with a reducing agent, such as carbon monoxide or hydrocarbons, to form nitrogen (N2) and water. This reaction also occurs on the surface of the catalyst and is facilitated by the presence of the precious metals.
- Adsorption: The pollutants are first adsorbed onto the surface of the catalyst, where they become attached to the precious metals. This increases the likelihood that the pollutants will react with the catalyst and be converted into less harmful substances.
Overall, the chemical processes that occur inside a catalyst work together to convert harmful pollutants into less harmful substances before they are released into the environment.
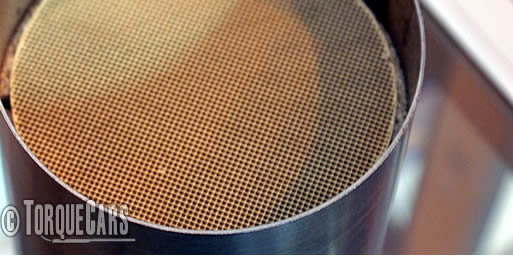
Above a standard Catalyst cross section and below a performance sports catalyst.
You can see the sports cat flows much better and is less restrictive.
Various grades of cell size are available between these two extremes.

The catalytic converter is a marvel of engineering design. When you turn on your engine it needs to cope with a rapid temperature rise of several hundred °C in less than a minute.
Operating temperatures are up to 1000°C, on a highly tuned TorqueCars members engine this becomes more frequently at the upper end of this range.
The catalyst needs to make good contact with the exhaust gases without impeding its flow.
The speed at which the exhaust gases flow means the catalyst has a matter of milliseconds to work its magic.
The answer is a ceramic lattice with around 400 to 1200 channels per square inch (62 to 186 cells per square cm) coated with a mixture containing the precious metals platinum, rhodium and palladium as well as other metal oxides.
Catalysts Can Be Damaged Easily
One of the main causes of damage to catalytic converters is high temperatures.
The usual operating temperature is 150-600°C, but misfiring and high driving speeds can lead to temperatures of up to 1000°C.
Unburnt fuel can damage a catalyst because it can cause an exothermic reaction on the surface of the catalyst, which generates high temperatures that can melt or otherwise damage the catalyst. This can occur because the unburnt fuel can accumulate on the surface of the catalyst and react with the oxygen in the air, which can cause a sudden increase in temperature.

In addition to damaging the catalyst, unburnt fuel can also decrease the efficiency of the catalyst by blocking the active sites where the chemical reactions occur. This can reduce the surface area available for reactions and prevent the catalyst from effectively converting pollutants into less harmful substances.
To avoid damage to the catalyst, it is important to ensure that the engine is running efficiently and that there are no issues with fuel injection or combustion. Regular maintenance, such as replacing worn spark plugs and fuel filters, can help to prevent unburnt fuel from entering the exhaust system and damaging the catalyst.
This damages the surface of the catalyst reducing its efficiency and in extreme circumstances can melt the ceramic.
Another problem is poisoning due to contaminants in the exhaust gases. Sulphur in the petrol or phosphorous from engine oil can both permanently damage the effectiveness of the catalyst.
Do Diesel Engines Use a Catalyst?
Many modern diesel engines will use a type of catalyst called a diesel oxidation catalyst (DOC) to reduce emissions of carbon monoxide (CO), hydrocarbons (HC), and particulate matter (PM) from the exhaust gases.
A diesel oxidation catalyst works by oxidizing the pollutants in the exhaust gas, similar to how a catalyst in a gasoline engine works. The DOC is typically made up of a ceramic or metallic substrate that is coated with a catalyst material, such as platinum or palladium. When the exhaust gas passes through the catalyst, the pollutants react with the precious metals on the surface of the substrate, converting them into less harmful substances.
In addition to diesel oxidation catalysts, some diesel engines also use other types of catalysts, such as diesel particulate filters (DPFs) and selective catalytic reduction (SCR) systems, to further reduce emissions of particulate matter and nitrogen oxides, respectively.
Please join us in our friendly forum to catch up with the latest trends in catalysts and exhaust systems for your car.
Please Check out my YouTube channel, we're regularly adding new content...
PLEASE HELP: I NEED YOUR DONATIONS TO COVER THE COSTS OF RUNNING THIS SITE AND KEEP IT RUNNING. I do not charge you to access this website and it saves most TorqueCars readers $100's each year - but we are NON PROFIT and not even covering our costs. To keep us running PLEASE Donate here
If you liked this page please share it with your friends, drop a link to it in your favourite forum or use the bookmarking options to save it to your social media profile.
Feedback - What do You Think?
Please use our forums if you wish to ask a tuning question, and please note we do not sell parts or services, we are just an online magazine.
Help us improve, leave a suggestion or tip
Please watch this video and subscribe to my YouTube channel.
6 Responses to “How car catalysts work and what they do.”

 Click to accept YouTube Cookies & Play.
Click to accept YouTube Cookies & Play.
Does Catalytic reduce the intake of gasoline in a car?
iT would be hard. Exhaust pipes are filled with black film dust smog which is very dirty and slemly. If you wore a gas mask and heavy duty gloves it may make the job easier. See your Jeep manual, it will tell you steps A-Z on how to remove it.
Dose catalist save fuel in vehicles. Please let me know
Does catalyst course high fuel consumption?
The only constructive comment I have has to do with ongoing tech. research which will hopefully soon find substitutes for the expensive, and profitable, precious metal catalysts currently in use; they are valuable & worth stealing. This has happened a lot in my area over the last 2 years. Apparently Rhodium, Platinum, & Palladium fetch good black market prices.
This was a very helpful article for my students. THank you.